
Experiment: Aldol Condensation
Week 7
- Is the product, tetraphenylcyclopentadienone,
more soluble in ethanol or toluene? Why?
- Referring to the carbon-NMR on page 34 of
your reader, can you account for the appearance of all of the
peaks?
- If a carbon-NMR of the product were taken
at very low temperatures (below -50 degrees Celsius), the appearance
of the spectrum would differ significantly from the one of p.
34 of your reader. Why?
- Would the presence of water at the start
of the reaction help or hinder the aldol condensation? Why?
- COLUMN CHROMATOGRAPHY:
By now you should understand the principles of Thin Layer Chromatography
(TLC). You know that TLC can help you identify the different
components present in your product mixture. You also know that
the distance that each component travels heavily depends on:
1) polarity of the components; 2) polarity of the solvent mixture.
Now imagine running a 3-dimensional TLC plate, but upside down.
Consider the following graphic:
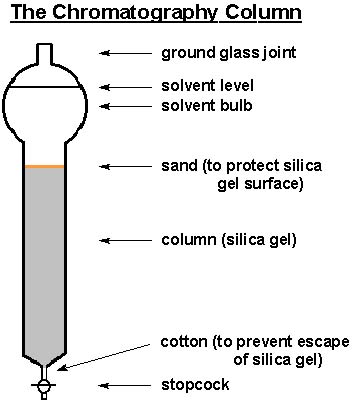

What you see above is a chromatography column. It is a glass
cylinder with a bulb and ground glass joint on top and a tapered
joint with stopcock at the bottom. Using the same principles
as TLC, the chromatography column can, with the utilization of
the proper amounts of silica gel, size of column (diameter and
length), and type of solvent system, separate various components
based on their respective polarities. With the aid of air pressure
from the top, one may collect solvent fractions from the column
that contain isolated components (purified).
This technique is a must for every experimental organic chemist
and is one of the most widely used ways to purify compounds from
undesired side products.
If you would like to know more about column chromatography, I'd
be more than happy to explain. E-mail me here.


